Energy vs. Power
Sometimes students use the terms 'energy' and 'power' interchangeably. However, it's important to note the differences...
Energy is the amount of heat or work that can be obtained by burning a certain amount of fuel. This is something that we can buy. For example, some units of energy are: kilowatt hours (kWh), British Thermal Units (BTU), Joules, barrels of oil, cords of firewood. In the US, your electric bill will usually read in kWh and your natural gas bill will read BTU's.
Power is the rate at which energy is used (converted from one form to another), or - it is the amount of energy needed to make the thing work. Power = energy over time. Some units of power are: Watts (W), Kilowatts (kW), horsepower, Btu/H, tons of cooling.
One helpful way to explain is to use water of an example. Picture someone carrying a bucket of water back from the well. That bucket of water represents the energy (the amount of [water] obtained from the well). The flow of the water out of the faucet represents the power (the rate at which that [water] is used). Here, the water bucket (energy) is measured in gallons and the flow (power) is measured in gallons per minute.
One (very inefficient) 100W light bulb can pull 100W of power. To figure out how much energy you're using, you need to add the time component. Say you use the fixture for 10 hours. That means you're using 100W x 10 hr = 1 kWh. (remember also that 1 kilowatt = 1000 watts). The average cost of electricity in the United States is about $.12.
Energy is the amount of heat or work that can be obtained by burning a certain amount of fuel. This is something that we can buy. For example, some units of energy are: kilowatt hours (kWh), British Thermal Units (BTU), Joules, barrels of oil, cords of firewood. In the US, your electric bill will usually read in kWh and your natural gas bill will read BTU's.
Power is the rate at which energy is used (converted from one form to another), or - it is the amount of energy needed to make the thing work. Power = energy over time. Some units of power are: Watts (W), Kilowatts (kW), horsepower, Btu/H, tons of cooling.
One helpful way to explain is to use water of an example. Picture someone carrying a bucket of water back from the well. That bucket of water represents the energy (the amount of [water] obtained from the well). The flow of the water out of the faucet represents the power (the rate at which that [water] is used). Here, the water bucket (energy) is measured in gallons and the flow (power) is measured in gallons per minute.
One (very inefficient) 100W light bulb can pull 100W of power. To figure out how much energy you're using, you need to add the time component. Say you use the fixture for 10 hours. That means you're using 100W x 10 hr = 1 kWh. (remember also that 1 kilowatt = 1000 watts). The average cost of electricity in the United States is about $.12.
Heat & Heat TransferHeat is a form of energy that can be transferred from one object to another (or even created at the expense of the loss of other forms of energy). In 'technical' terms, it is "the measure of the average kinetic energy of the particles in a sample of matter, expressed in terms of units or degrees designated on a standard scale". It is measured on a number of scales, including Kelvin, Fahrenheit and Celsius
remember that matter is always seeking equilibrium - so if a colder material exists, heat will flow from hot to cold. (If you're curious, the equation of heat flow is given by Fourier's Law of Heat Conduction). Remember also that heat can flow through convection, conduction and radiation. Heat flow is commonly measured in Joules, BTU (amount of energy needed to heat 1 lb. of water 1°F (US)) or Cal (Calorie – amount of energy needed to heat 1 gram of water 1°C (Metric)) |
Solid, Liquid and Gas
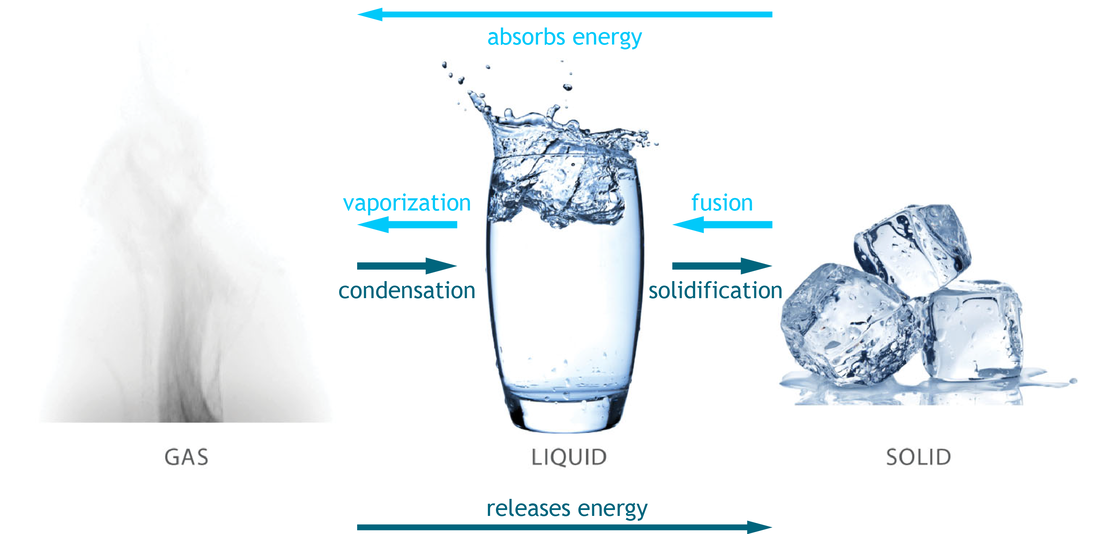
Matter occurs in three phases, liquid, solid and gas. (Technically, there's a fourth phase - plasma - but it's a bit beyond the scope of this class. As matter changes from phase to phase, it either absorbs or releases energy. Consider a pot of water, for example - for some reason, you decide to boil a pot of ice cubes. As heat is applied, that water changes from the solid form, to liquid and eventually to gas (steam). This process absorbs the heat (energy) of the stove. Conversely, when the process is reversed, gas (steam) to liquid to solid (ice), releases energy. This phenomenon is shown more clearly in the diagram below.
In each of the different phases, the water stores or releases energy in its chemical bonds. It's important to know the direction of heat and energy flow here in order to capitalize on this stored energy.
In each of the different phases, the water stores or releases energy in its chemical bonds. It's important to know the direction of heat and energy flow here in order to capitalize on this stored energy.
Latent & Sensible Heat
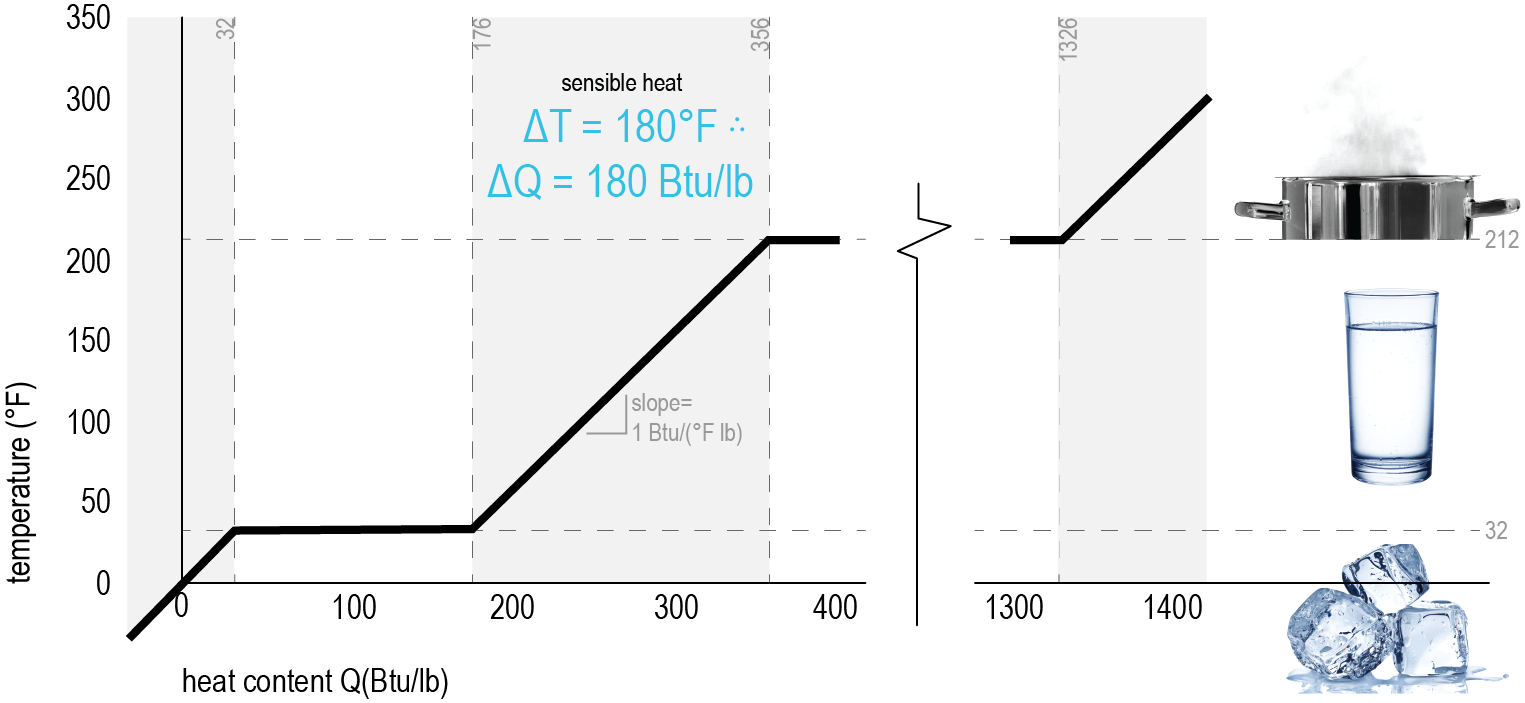
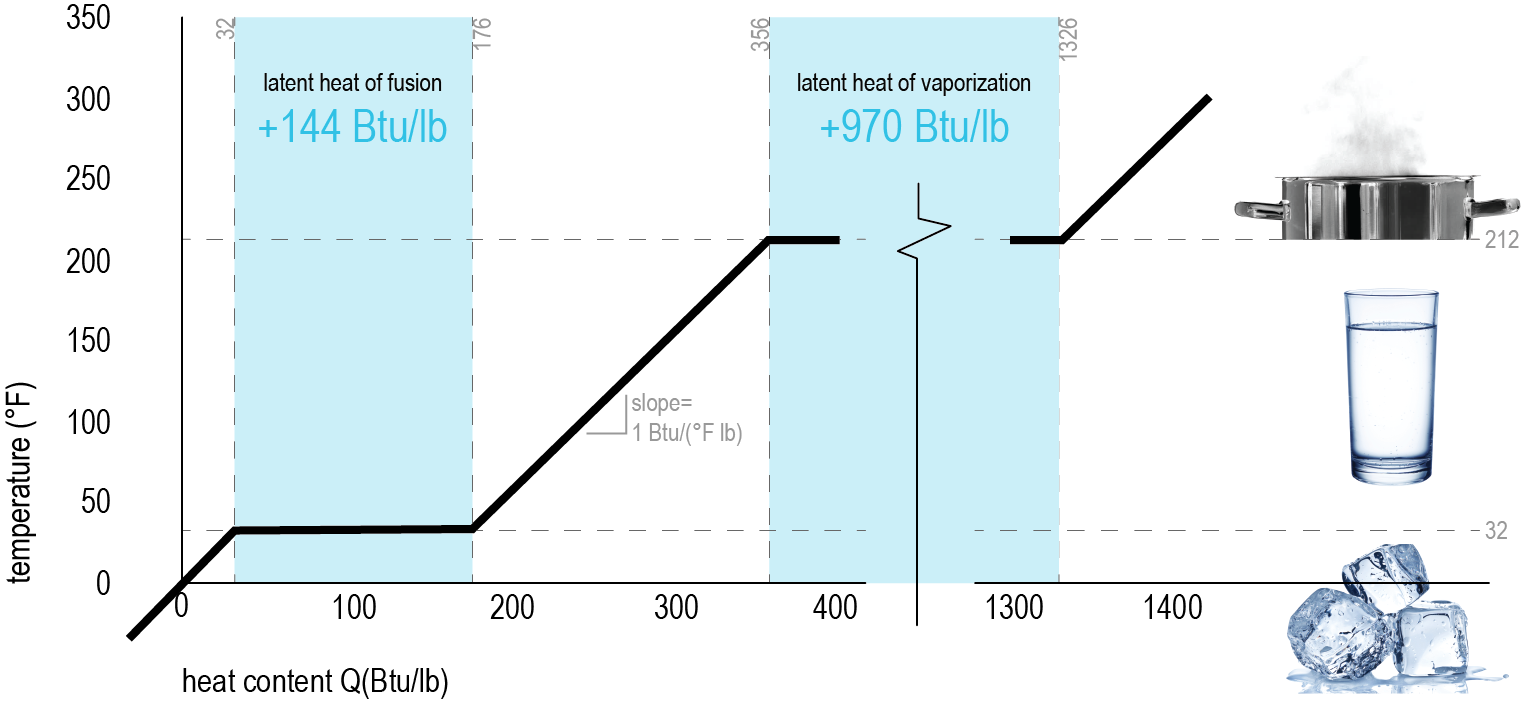
These two graphs are a helpful way to understand the difference between sensible and latent heat. With temperature on the vertical axis and heat content (energy) on the horizontal, you can see the relationships between temperature rise and heat (energy) gain.
|
We already know that 1 BTU is the amount of energy that it takes to heat one pound (lb) of water by 1°F. Sensible Heat (shown left in grey) represents the changes in the temperature system without changing anything else (phase, pressure, volume, etc). It is the heat that you can feel. Latent Heat (shown left it blue) is the energy stored in the chemical bonds - it is only released during a constant temperature process (phase change). It causes a change in energy without changing the temperature |
Phase Change Materials
Now that we understand the different phases and different heat types in various materials, we can start to understand phase change materials (PCM's) and how they can be used in architecture. These materials are capable of storing and releasing large amounts of energy, also known as Latent Heat Storage units (LHS). The primary benefit of PCM's depends on latent heat storage - a change in heat without a change in temperature. Even a small change in temperature can be used for storing energy or releasing energy that was already stored.
Can you think of a few different phase change materials?
Can you think of a few different phase change materials?
Phase change materials are all around us. Shown above: Paraffin (candle wax), ice (water) and CHOCOLATE. Any material that changes phase (regardless of temperature) is a PCM. It's important for us to figure out which of these materials are useful to buildings. Most notably - the heat of fusion (melting). Consider two different materials - coconut oil (below left) and steel (below right) - both occur in solid form, but can turn to liquid at dramatically different melting temperatures - 76°F and 2500°F, respectively. The melting temperature of coconut oil, while within the range of human comfort conditions, is likely too low to be useful in buildings. Meanwhile, the incredibly high temperature of melting steel is well beyond human comfort conditions and is also not useful to conditioned spaces in buildings. The characteristics required for effective and predictable thermal energy storage excludes a large number of materials.
In modern construction, building enclosures are made as lightweight as possible. Storrs Hall, for example, is built with brick and heavy masonry - the walls are quite thick in some places. Meanwhile, the new buildings on campus are built with metal stud-framed walls and brick veneer - with minimal thickness. These new buildings have little-to-no thermal mass, meaning they may overheat in the summer and can't retain heat in the winter. Often, heating and cooling systems are installed to maintain temperatures within the comfort zone. However, it is also possible to replicate the effect of thermal mass of the building using phase change materials (PCM).
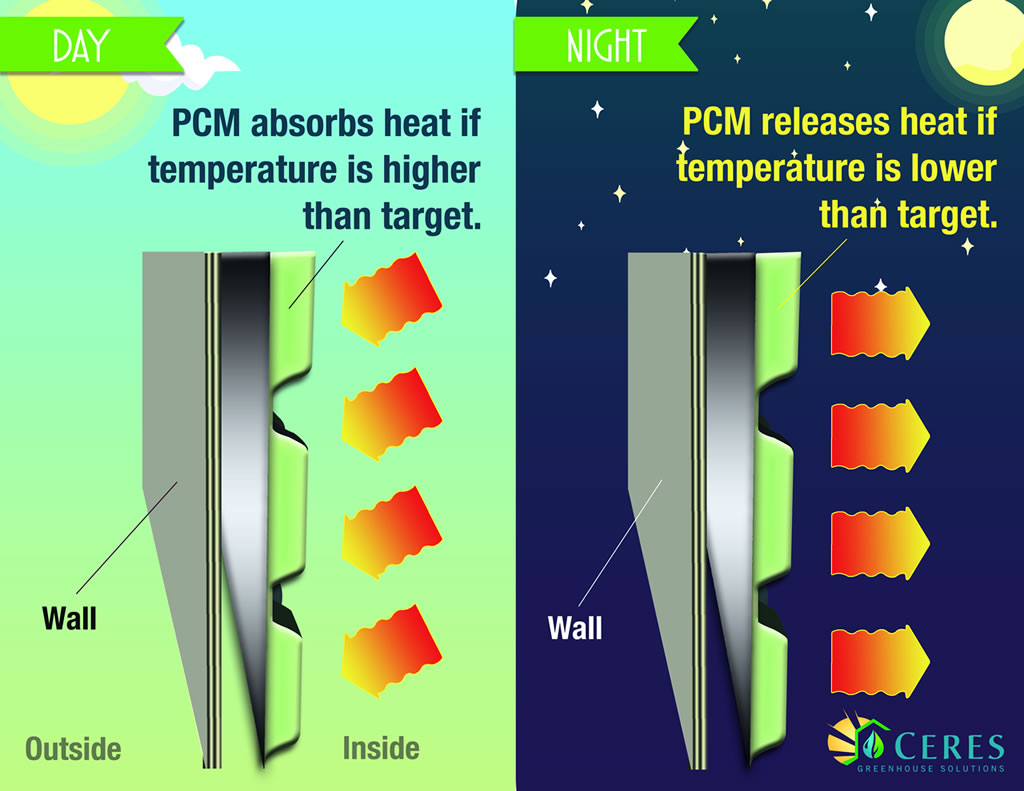
Phase change materials can operate in much the same fashion as thermal mass... but without the mass. Below are examples of commercially available products that can store the excess heat during the day and release it at night, or vice versa.
Phase change materials can operate in much the same fashion as thermal mass... but without the mass. Below are examples of commercially available products that can store the excess heat during the day and release it at night, or vice versa.
Heat Capacity
|
According to Kiel Moe, "the coupling of thermal comfort with convective air flows for human comfort is as old as fire". This comfy cat is enjoying the direct radiation from the fire while the heat transfers throughout the space via convection. The fireplace, or the hearth, was often the center of a dwelling so that all spaces could benefit from its heat. However, as conditioning technology evolved, the fuel source was separated from the delivery of heat. Take the modern radiator for example - heat is 'piped' from the source to the distribution zone. The human body decouples its thermal conditioning system (blood flow, vascular system) from its ventilation system (breath, respiratory system). Water is 832 times denser than air (meaning it also has a higher energy density (capacity to hold hear) than air. As such, air is a poor medium for capturing and channeling heat energy ...so why do we heat and cool our buildings with air, not water?? |
Energy density = the amount of energy that can be stored in unit mass
Specific heat = the amount of energy required to raise a unit volume of a material by one degree, an indication of a material’s capacity to capture and channel energy
Volumetric heat capacity = Describes the ability of a given volume of a substance to store internal energy while undergoing a temperature change (without undergoing a phase transition)
Specific heat = the amount of energy required to raise a unit volume of a material by one degree, an indication of a material’s capacity to capture and channel energy
Volumetric heat capacity = Describes the ability of a given volume of a substance to store internal energy while undergoing a temperature change (without undergoing a phase transition)
Separate the provisions of thermal conditioning
...and fresh air
The human body needs both thermal conditioning and fresh air. So when you move to a water-based (radiant) system, you need to provide the fresh air by other means.
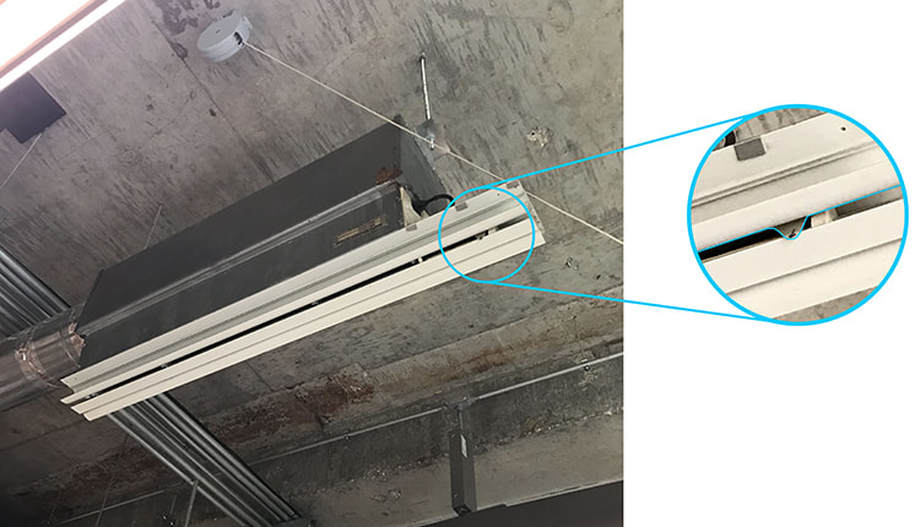
In the photo below, this diffuser provides both heat/cooing AND fresh air. So when we were cold (which we often were), we'd adjust the switch (shown in blue below) to close the vent. HOWEVER, this meant that we wouldn't get any fresh air (aside from the air leaking in through the envelope). Keep this in mind as you design your next HVAC system.
In the photo below, this diffuser provides both heat/cooing AND fresh air. So when we were cold (which we often were), we'd adjust the switch (shown in blue below) to close the vent. HOWEVER, this meant that we wouldn't get any fresh air (aside from the air leaking in through the envelope). Keep this in mind as you design your next HVAC system.
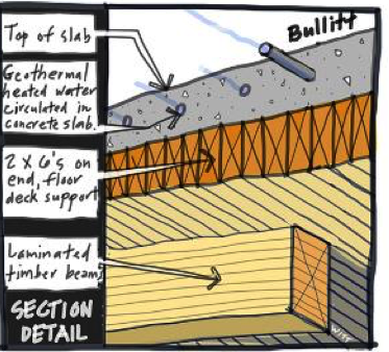
Here's a photo of the Bullitt Center in Seattle. This building is one of the first Living Building Challenge buildings in the country and is incredibly low energy. One way they were able to achieve this was by separating thermal comfort from fresh air. You can see only one small duct running through the left side of the photo to provide fresh air. Because it's moving at a low volume and a mild temperature, this duct can be quite small.
The next project is the 3-for-2 Project, a study that explored fitting 3 usable office floors into the same space as 2 floors using traditional means. With land and space at a premium in the island-city of Singapore, this method proposes an increase in building efficiency from many aspects.

3-for-2: Less energy, more space. ETH Zurich
Conventional air conditioning uses large amounts of cold, dry air to achieve thermal comfort. It also uses very low temperatures to dehumidify the air. This system employs several approaches:
• Splits cooling (sensible cooling) and dehumidifying (latent cooling)
• Cooling can use much higher temperatures (18C, ~64F) – reduces energy use by 40%
• Uses radiative heat transfer (cold surfaces) – (challenge to avoid condensation that might occur when hot-humid air comes into contact with colder surfaces)
• Uses water instead of air for heat transport (water has greater heat-transporting capacity) – smaller pipes – 20% additional office space
• Decentralized ventilation – at the façade
• Reduces construction materials by 16%
• Splits cooling (sensible cooling) and dehumidifying (latent cooling)
• Cooling can use much higher temperatures (18C, ~64F) – reduces energy use by 40%
• Uses radiative heat transfer (cold surfaces) – (challenge to avoid condensation that might occur when hot-humid air comes into contact with colder surfaces)
• Uses water instead of air for heat transport (water has greater heat-transporting capacity) – smaller pipes – 20% additional office space
• Decentralized ventilation – at the façade
• Reduces construction materials by 16%
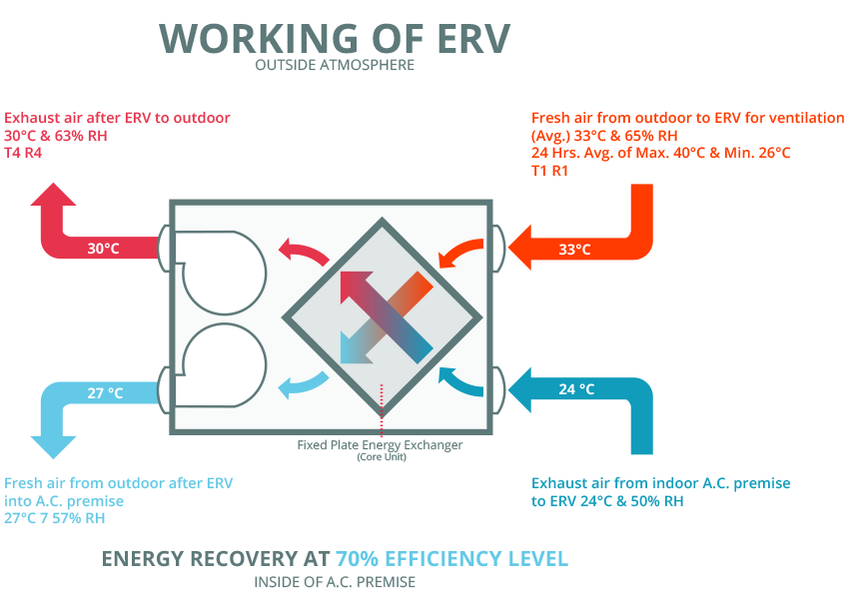
Energy Recovery & Heat Exchange
In most low-energy buildings, you'll probably find some sort of air-to-air heat exchanger. This system works to keep all the conditioned energy inside the building, while passing all the stale air out. This way, you can recover some of the heat that would otherwise be wasted as exhaust air.
Breathing Walls
This project by Salmaan Craig and Jonathan Grinham was part of the inspiration behind this class. It's a great example of how architects can reclaim some of the thermal controls that have been relegated to the engineer. I recommend everyone read it here.
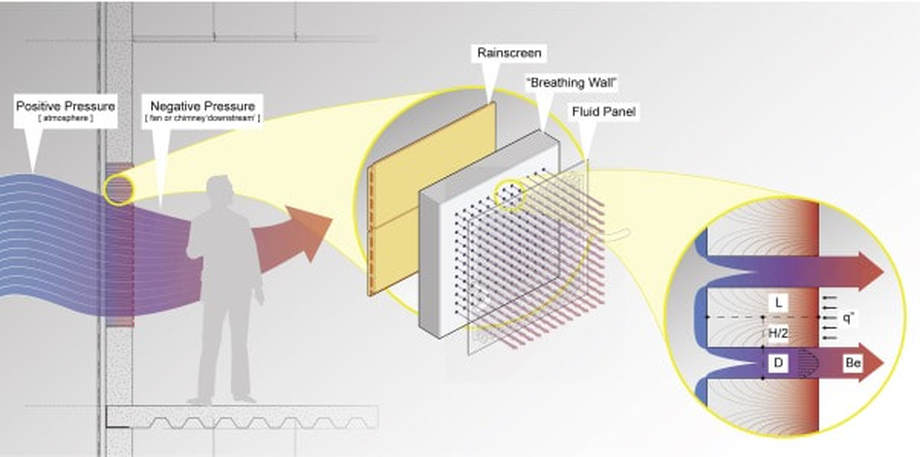
Image by Craig and Grinham (2017)
|
(a) plan view schematic
Image by Craig and Grinham (2017) |
(b) experimental setup
Image by Craig and Grinham (2017) |
|
Their inspiration actually came from turbine blades! They looked at the porous blade for a gas-turbine engine. The paper gives a method to optimize the size and spacing of the cooling channels. The idea was to flood the inside of the blade with water or air at high pressure. And as the fluid or gas transpires through the pores, it prevents the blade from overheating. |